Are you looking for the trick to find a formal charge or calculate the formal charge on any atom in a molecule? In this article, you will learn the trick or method to calculate the formal charge on an atom.
Table of Contents
What Is Meant By Formal Charge:
The formal charge is the charge denoted to an atom in a molecule. So, what is the need for a formal charge? Formal charge decide which lewis acid is the best to structure.
Formal charges area unit calculated by employing a set of rules and area unit helpful for accounting for the electrons once writing a reaction mechanism.
What is the Role of Formal Charge:
How we can determine the stability of structure based on the formal charge.
It’s simply less the number of formal charges more the stable structure.
However, they don’t have any intrinsic physical which means. They will even be used for qualitative comparisons between different resonance structures of the identical molecule.
Sometimes have identical signs. Because of the partial charge of the atom, however, there are unit exceptions.
How to calculate the formal charge?
A formal charge of an atom can be found with the help of the following formula.

As we discussed above, a formal charge is calculated of atoms from a molecule or ion. Then we can easily find a total formal charge on any molecule or structure.
Before Go ahead you can watch this video, which is explained by Suhani Ma’am our Cofounder. This video will clarify the two examples which we are going to explain here.
For example, we can find the formal charge of sulphur atom (S) from sulphate ion (SO42-)
Examples 1:
Let’s find the formal charge of the sulphate ion (SO42-).

To find the total formal charge of sulphate ion (SO42-) we need to find the formal charge of all atoms individually.
Firstly, we will find the formal charge on the sulphar (S) atom.
According to the above formula to calculate the formal charge.
Sulfur is belonging to group number 16 so, the valence electrons are 6
You can see in the structure of sulfate ion there is no non-bonded pair of electrons. Therefore the non bonded electrons are zero
In the structure, there are six bond lines around the sulfur atom. Therefore bond lines are 6.
List here:
Valence electrons in sulfur atom = 6
Non Bonded atoms in Sulphur = 0
No. of. Bond Lines = 6
By using the formula of formal charge
Formal charge on S = 6 – 0 – 6 = 0
In this way, the formal charge on sulphur is 0 (zero)
Similarly, calculate the formal charge on oxygen atoms.
There are 4 oxygen atoms are presented in the structure of sulfate ion.
For our convenience, we will give the number to the oxygen atoms likewise.

We need to find the formal charges of O1, O2, O3, and O4
But we noticed here that the formal charges on O1 and O3 are the same because the O1 and O3 are identical atoms.
Now, we will find, the formal charge on the O1 atom.
Collect the data:
No. of. Valence electrons: 6
No. of. Non bonded electrons: 6
No. of Bond Line: 1
Therefore by formula:
Formal charge on O1 = 6 – 6 – 1 = 6 – 7 = -1
As we discussed, O1 and O3 are identical so, formal charges are -1
In the same way, the formal charges on O2 and O4 are the same due to the identical nature of the oxygen atoms.
Similarly, the formal charge on O2
Collect the data:
No. of. Valence electrons: 6
No. of. Non bonded electrons: 4
No. of. Bond Line: 2
Therefore according to formula:
The formal charge on O2 = 6 – 4 – 2 = 6 – 6 = 0
O2 and O4 are identical, so their formal charges are 0 respectively.
So, calculated formal charges on each atom
S = 0
O1 = -1
O2 = 0
O3 = -1
O4 = 0
According to all formal charges above, after the addition of all formal charges of atoms. We can say that the net formal charge on sulfate ion is -2.
Example 2:
Find the formal charge on ozone molecule

To calculate the net formal charge on the O3 molecule, we will calculate the formal charge of each oxygen atom.
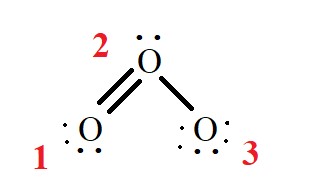
O1, O2, O3 are the three oxygen atoms. We have to find the formal charge for each oxygen atom.
For O1 :
No. of. Valence electrons = 6
No. of. Non bonded electrons = 4
No. of. Bond Line = 2
By using formula
The formal charge on the O1 atom = 6 – 4 – 2 = 6 – 6 = 0
For O2:
No. of. Valence electrons = 6
No. of. Non bonded electrons = 2
Bond Line = 3
The formal charge on O2 atom = 6 – 2 – 3 = 6 – 5 = 1
For O3:
No. of. Valence electrons = 6
No. of. Non bonded electrons = 6
Bond Line = 1
Therefore by formula of the formal charge on O3 atom = 6 – 6 – 1 = 6 – 7 = -1
O1 = 0
O2 = 1
O3 = -1
After the adding all formal charges of oxygen atoms we will get 0 (zero) as formal net charge on O3 molecule.
In this way, you can find any formal charge of an atom of molecules or net formal charge. You have to just know the lewis structure of the molecule.
I hoped you loved this method of how to calculate formal charge of any molecule from Lewis structure. If you liked then follow us on YouTube, Facebook and Instagram for more connection with us.
